Polarity Chart Electronegativity
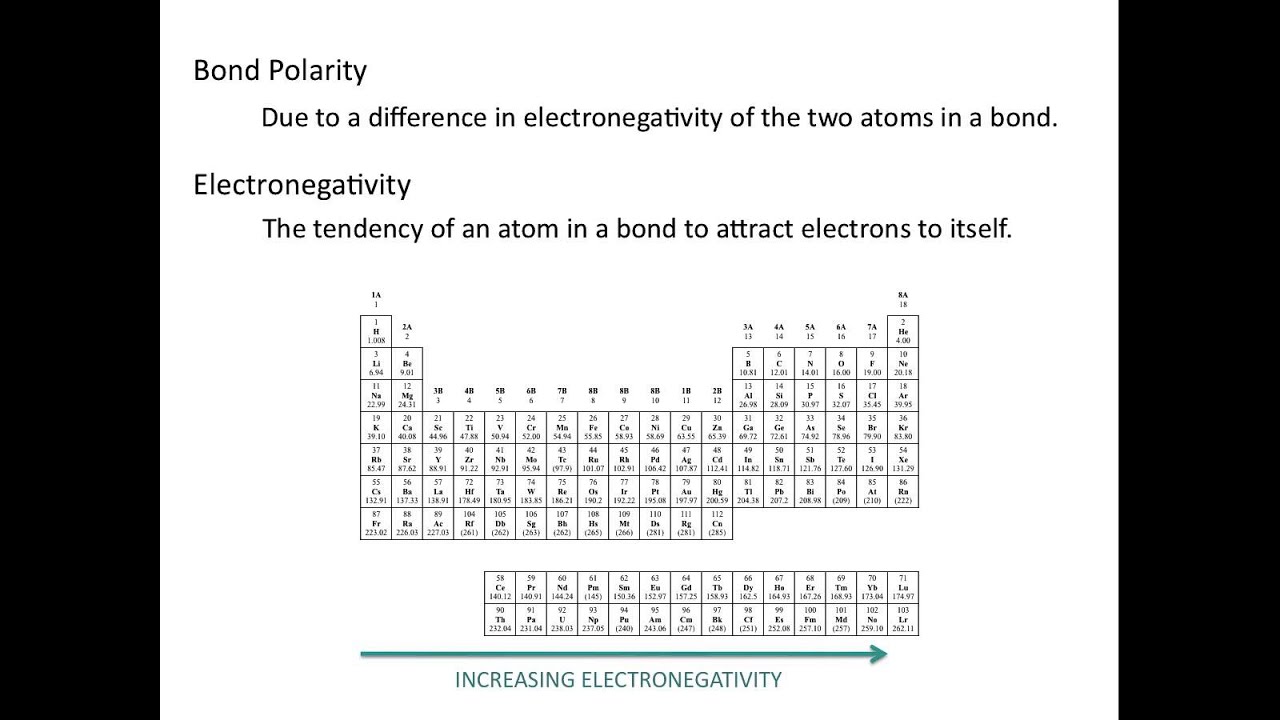
Electronegativity and polar covalent bonding.

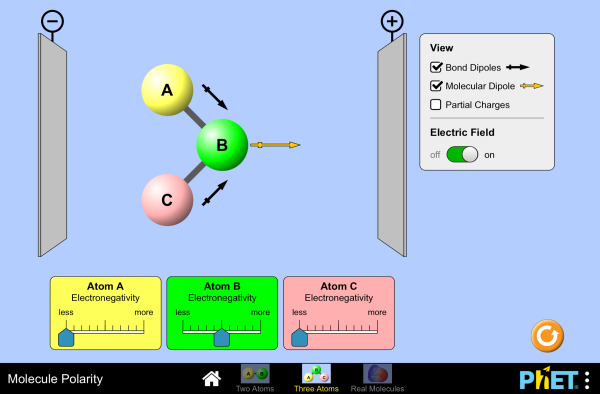
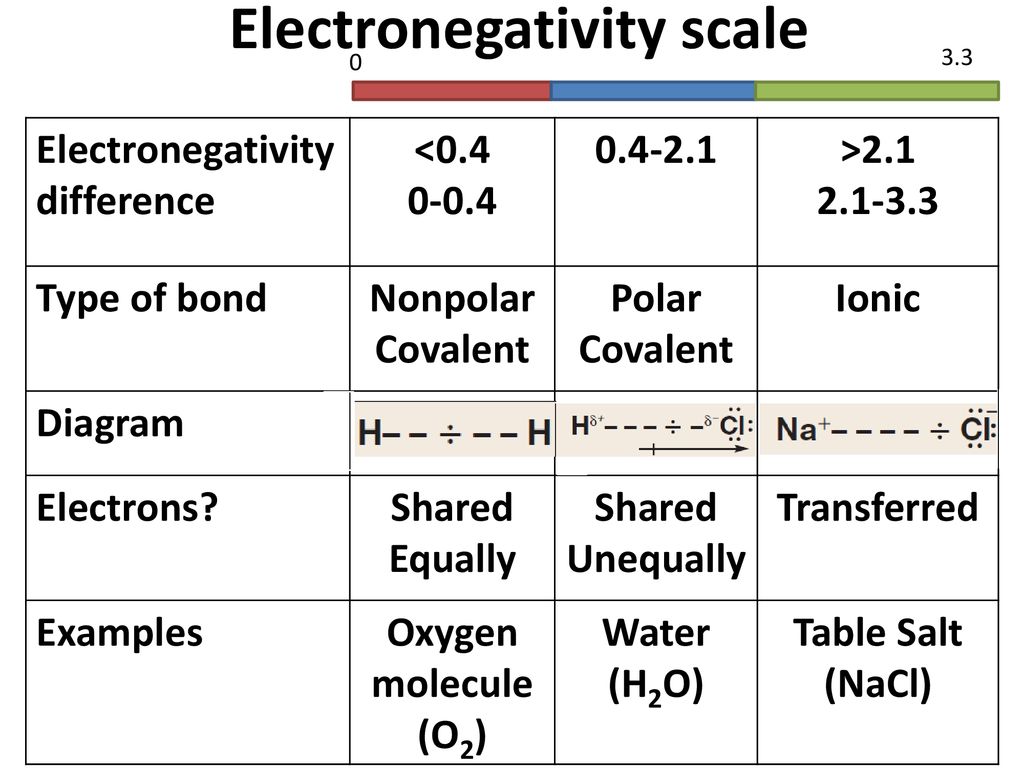
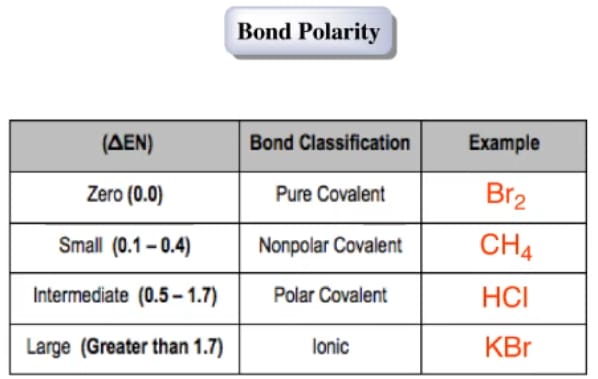
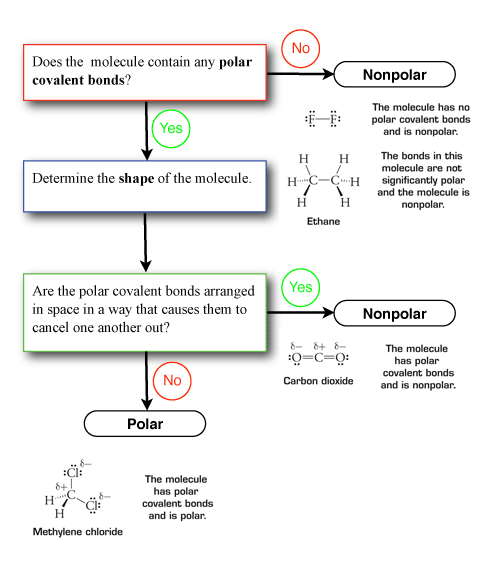
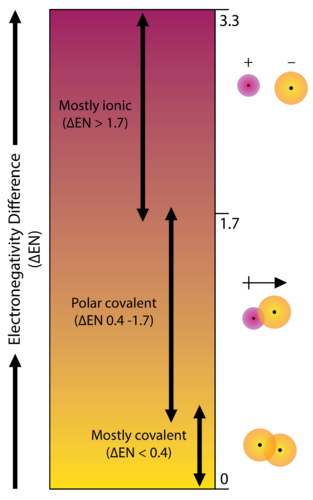
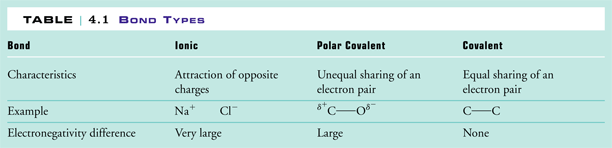
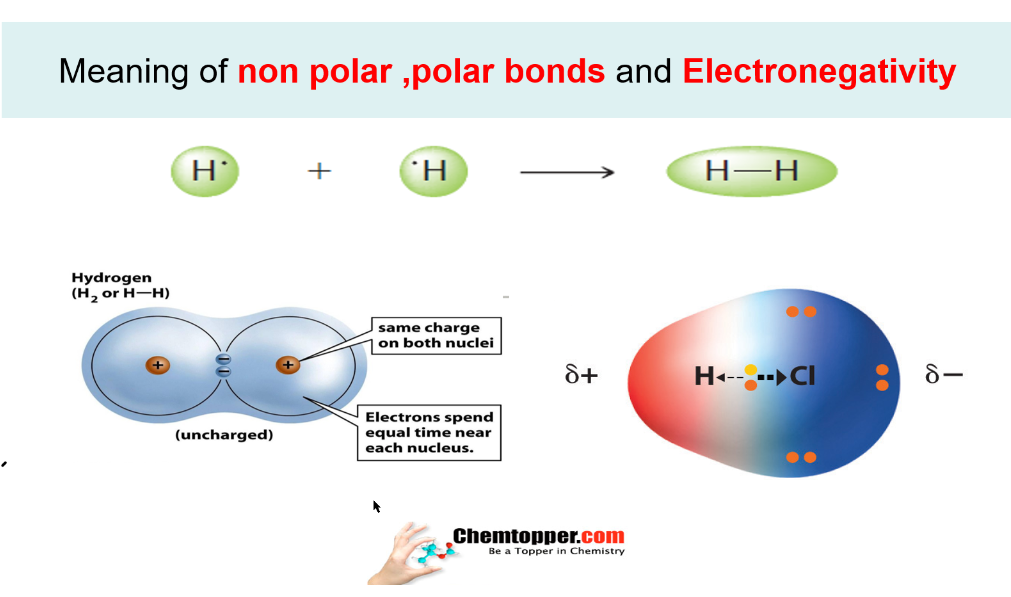
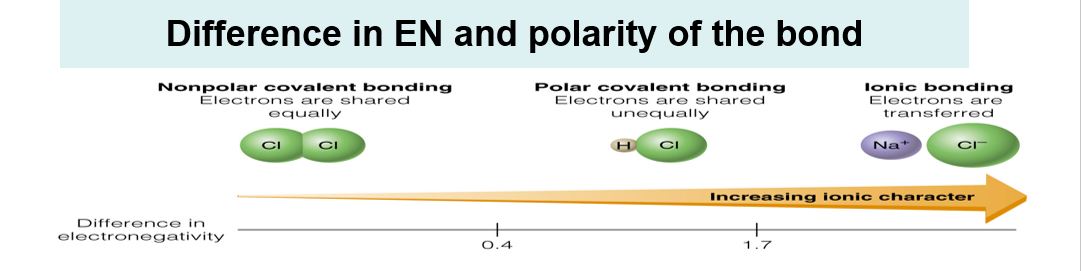
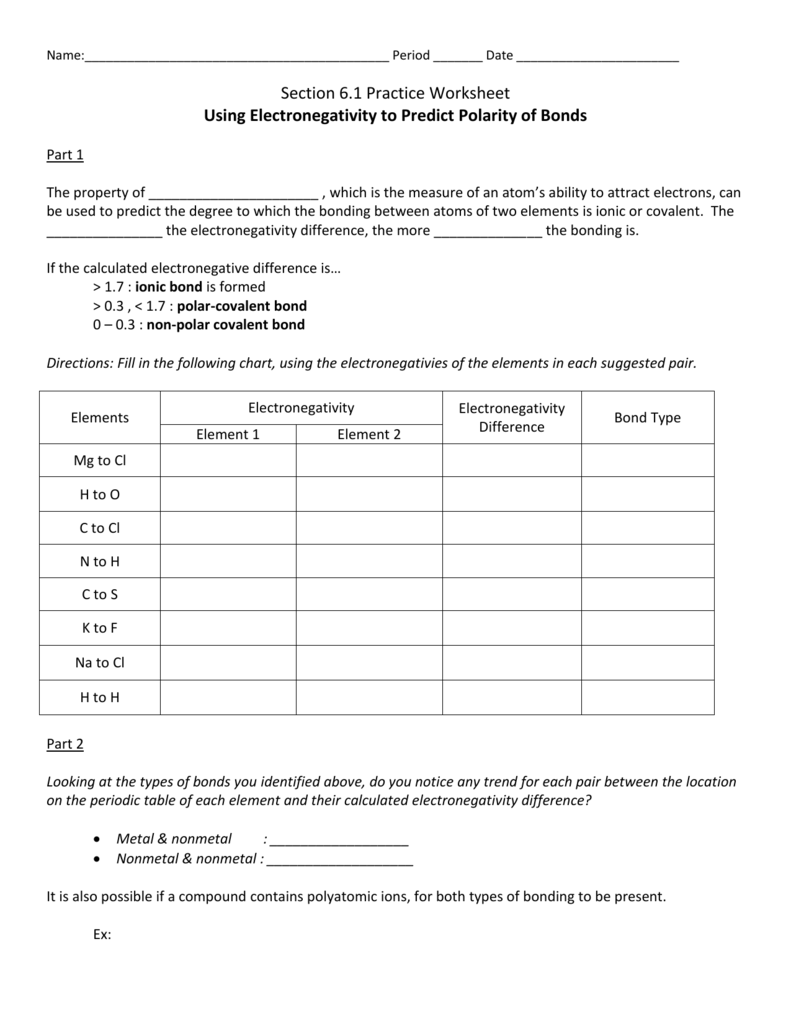
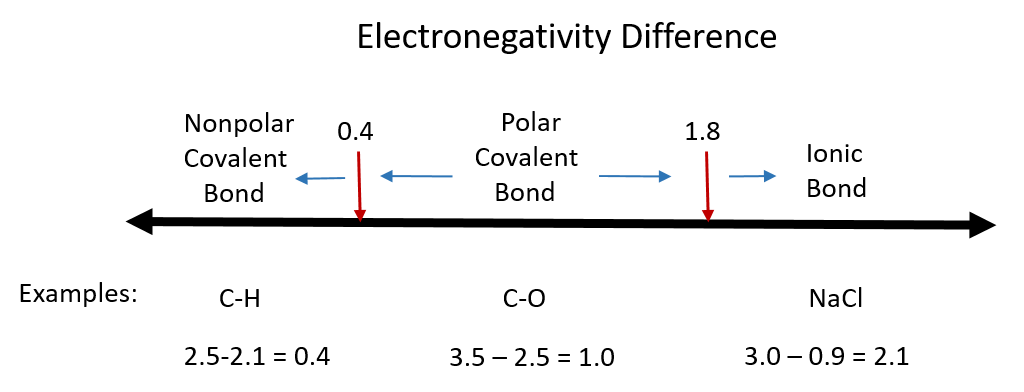
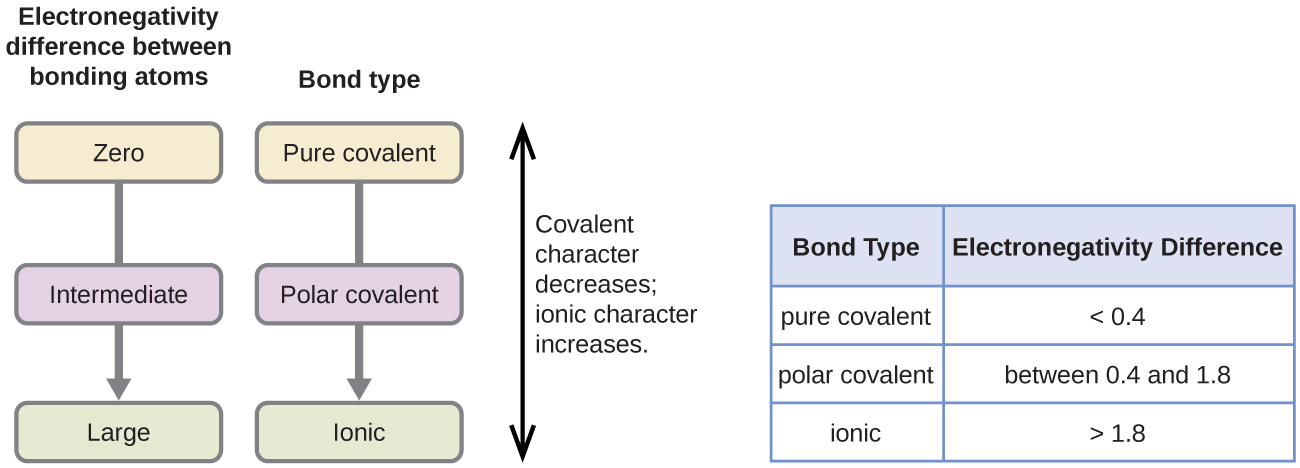
Polarity chart electronegativity. When a chlorine atom covalently bonds to another chlorine atom the shared electron pair is shared equally. When the difference is very small or zero the bond is covalent and nonpolar. To predict if the resulting molecule will be polar or nonpolar.
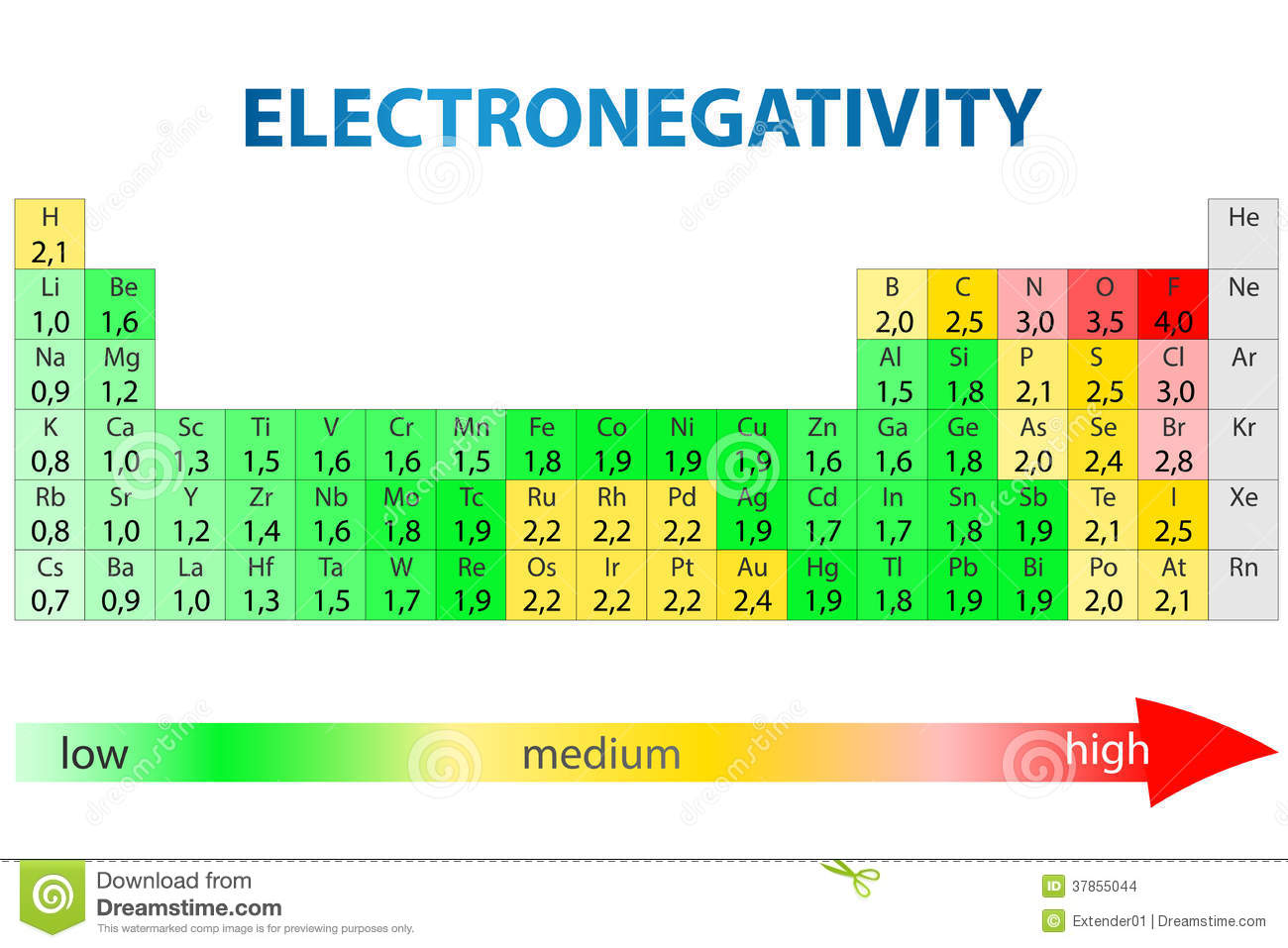
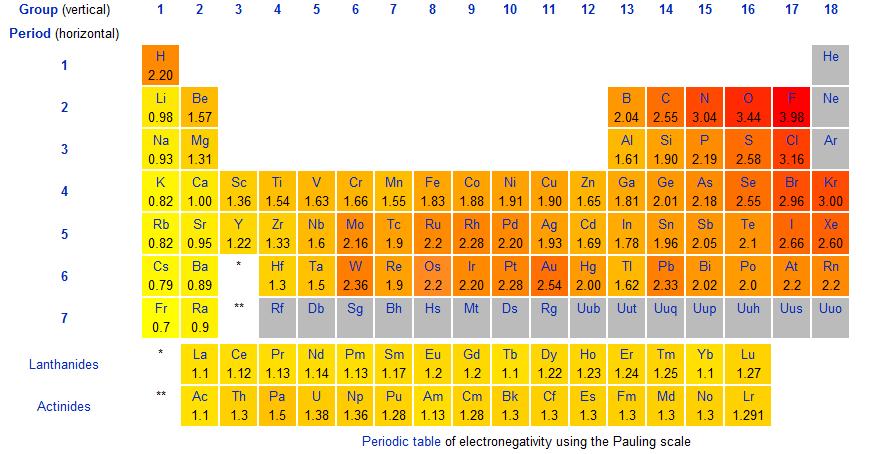
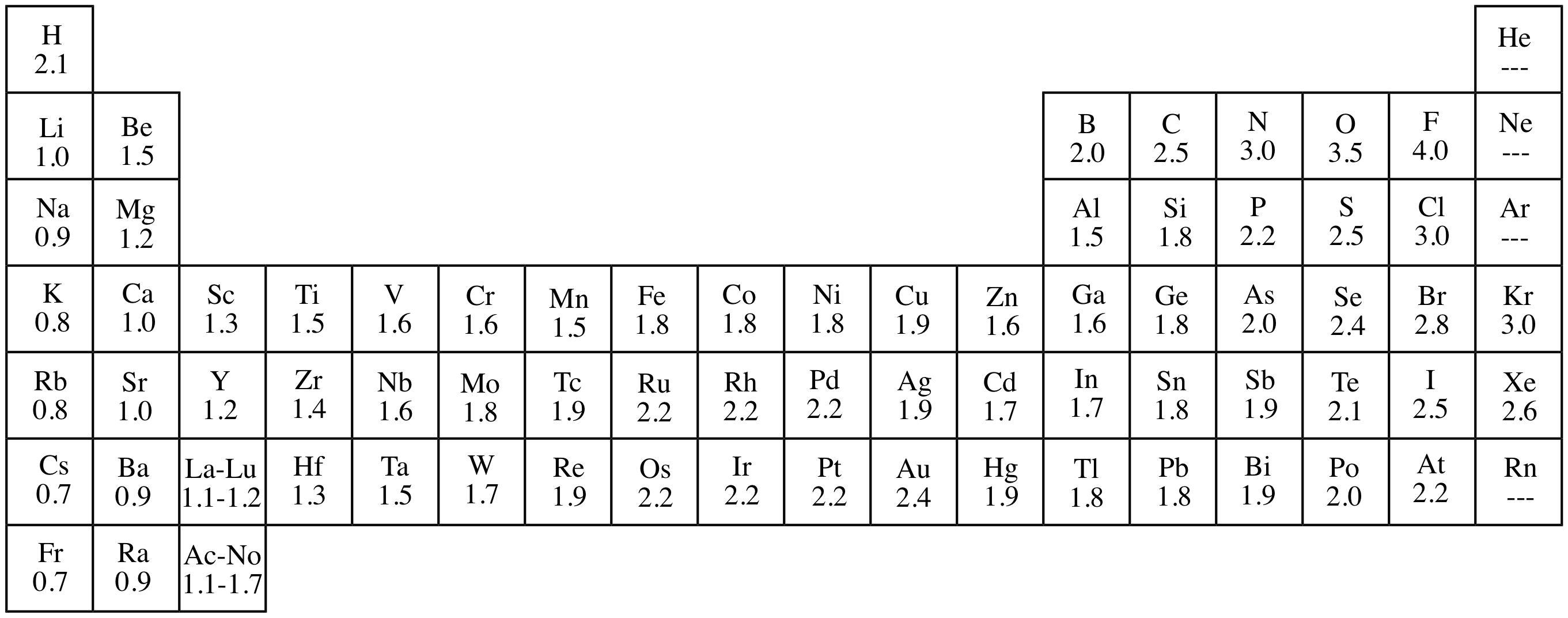
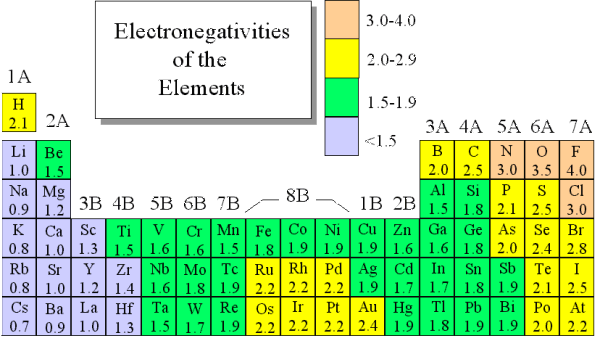
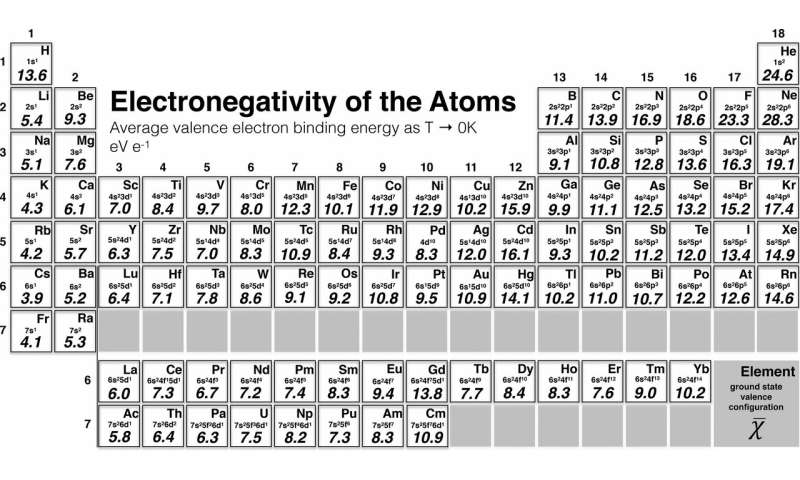
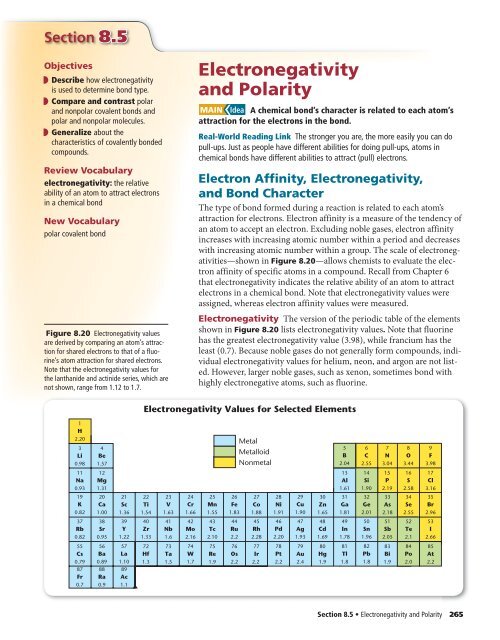
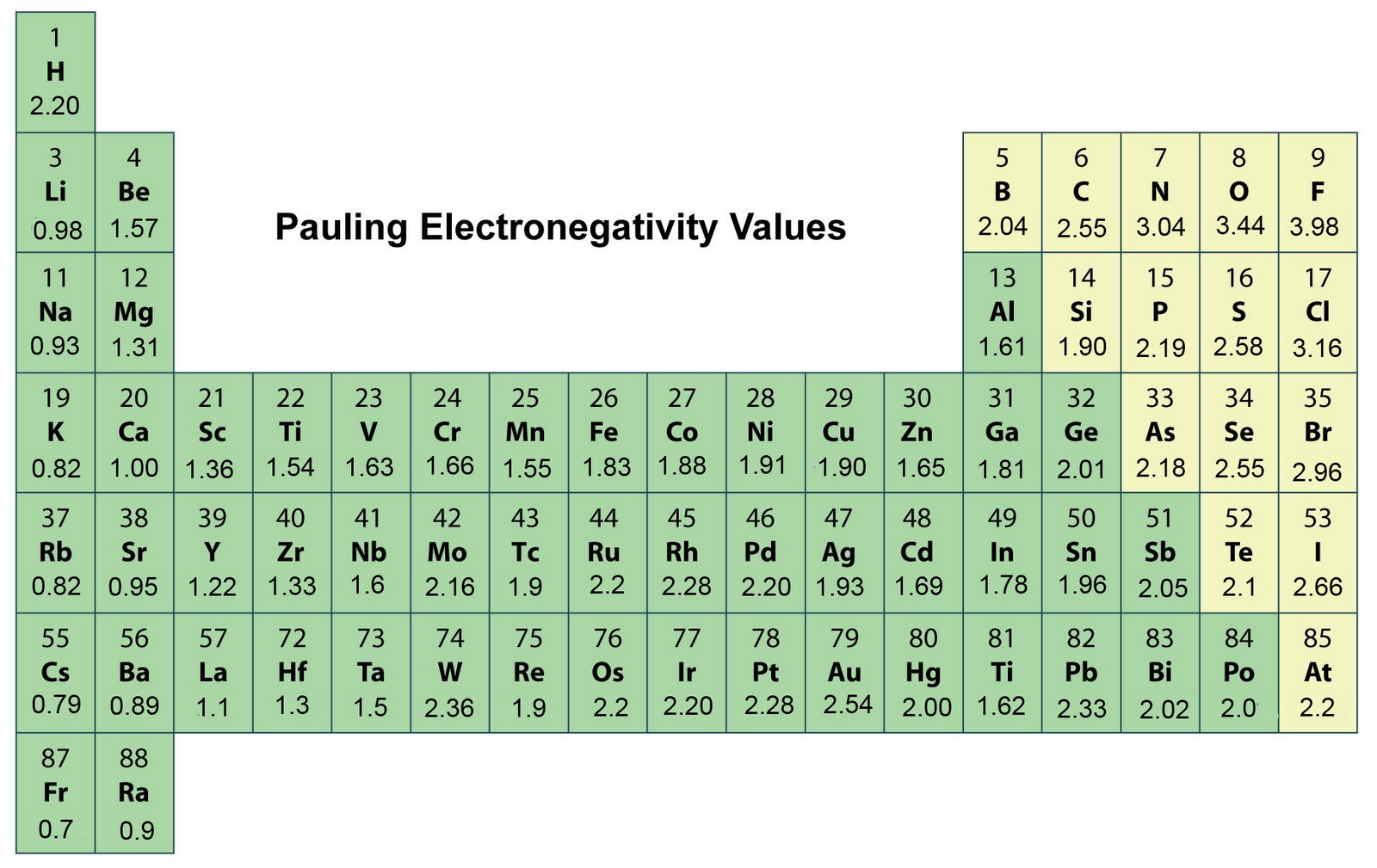
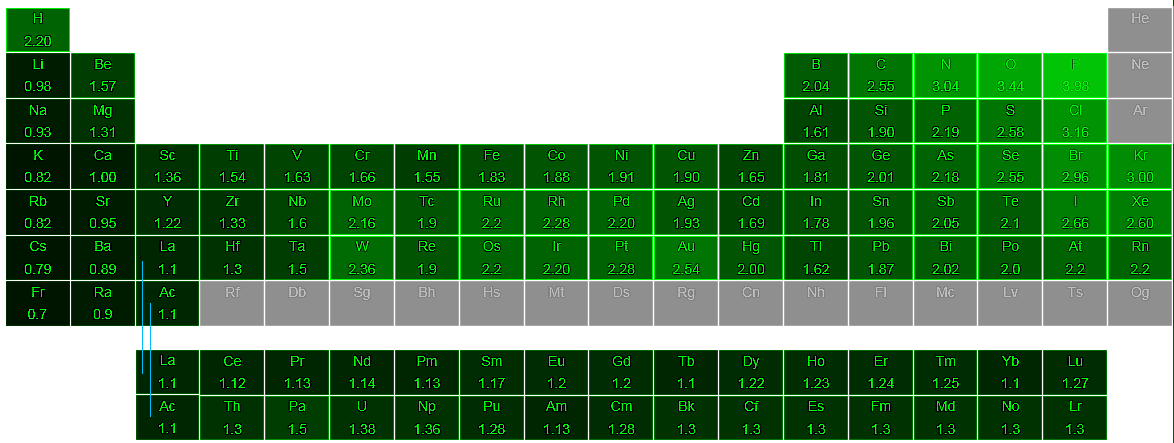
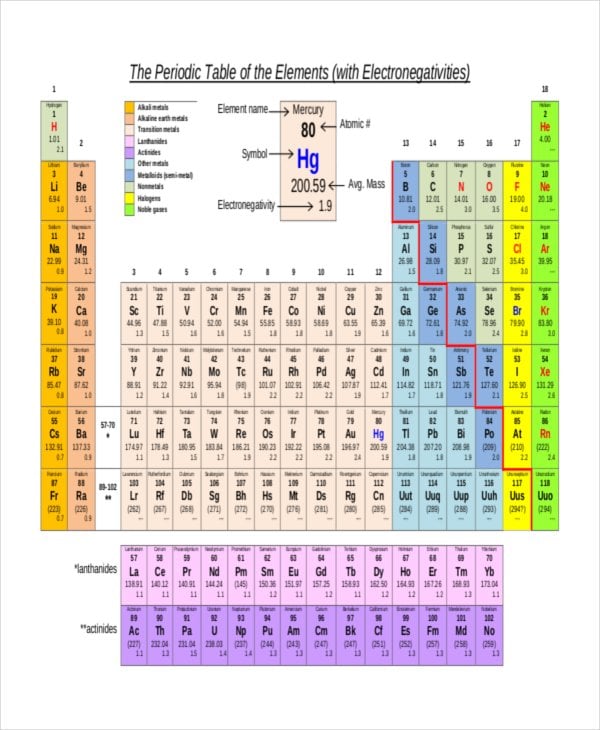
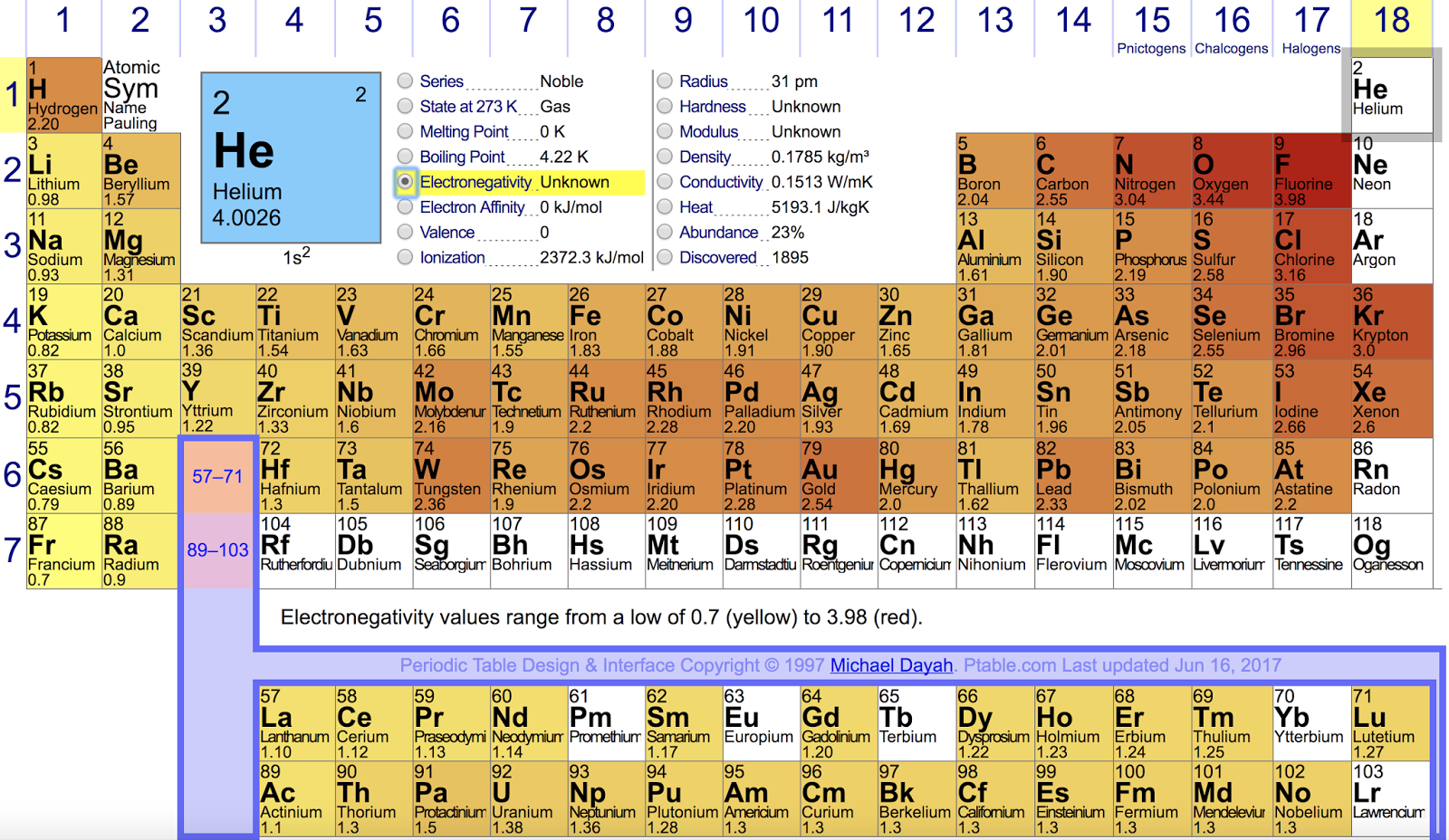
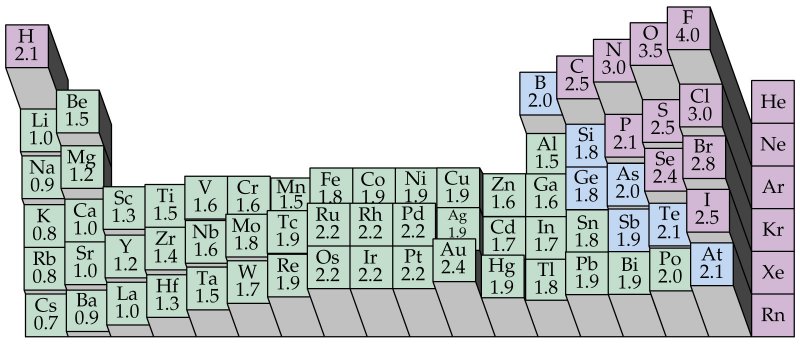
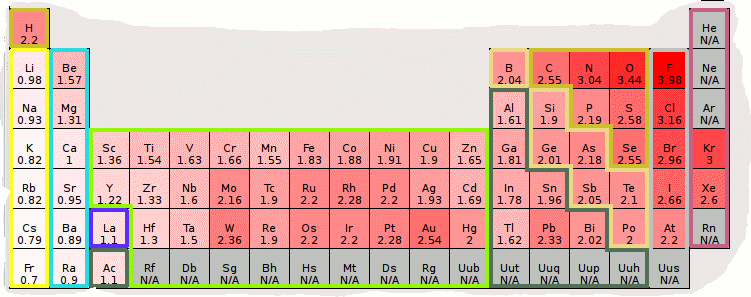
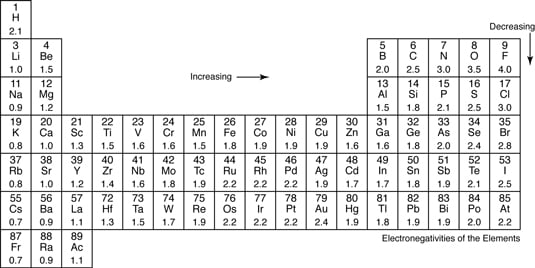
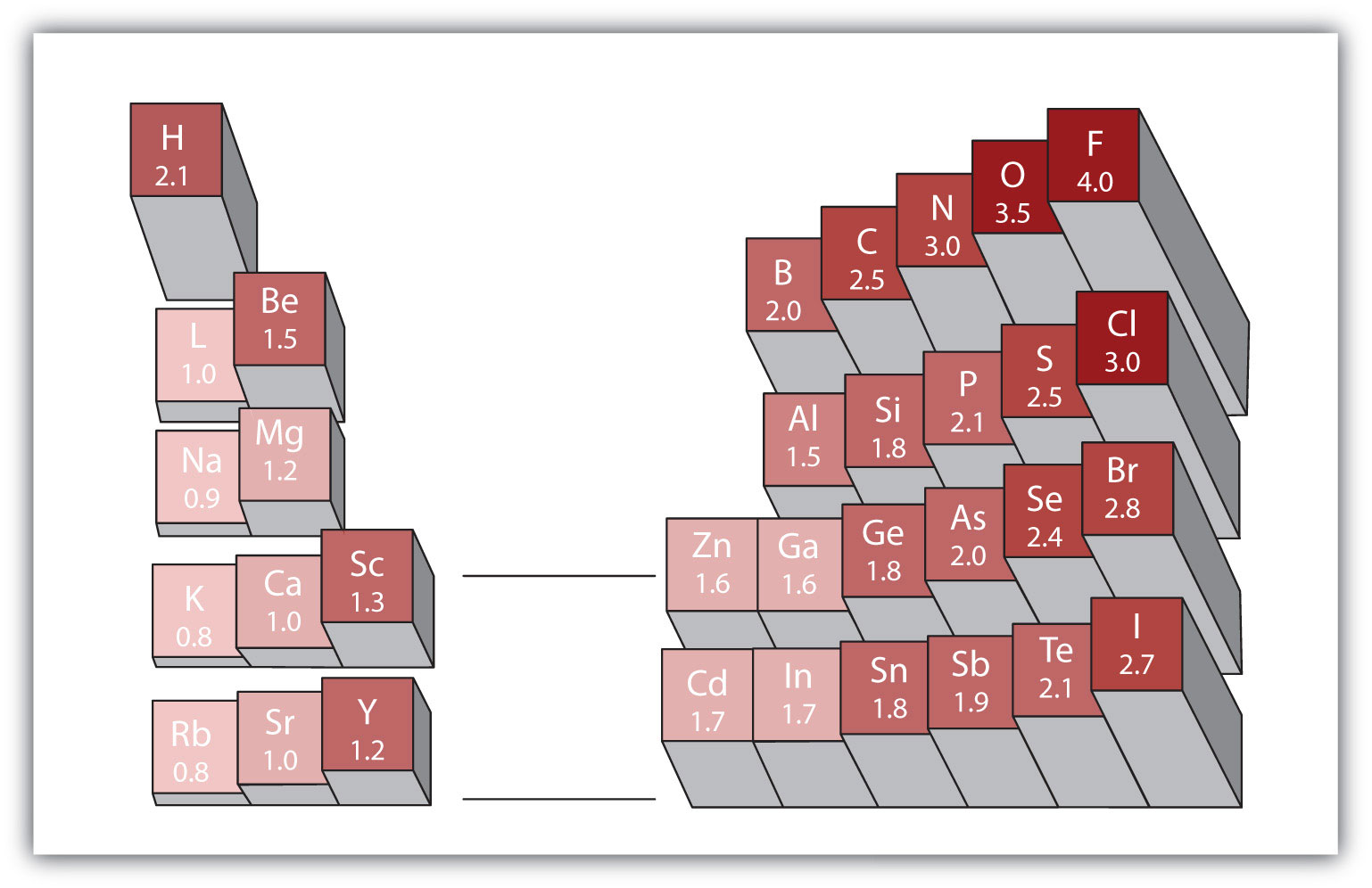
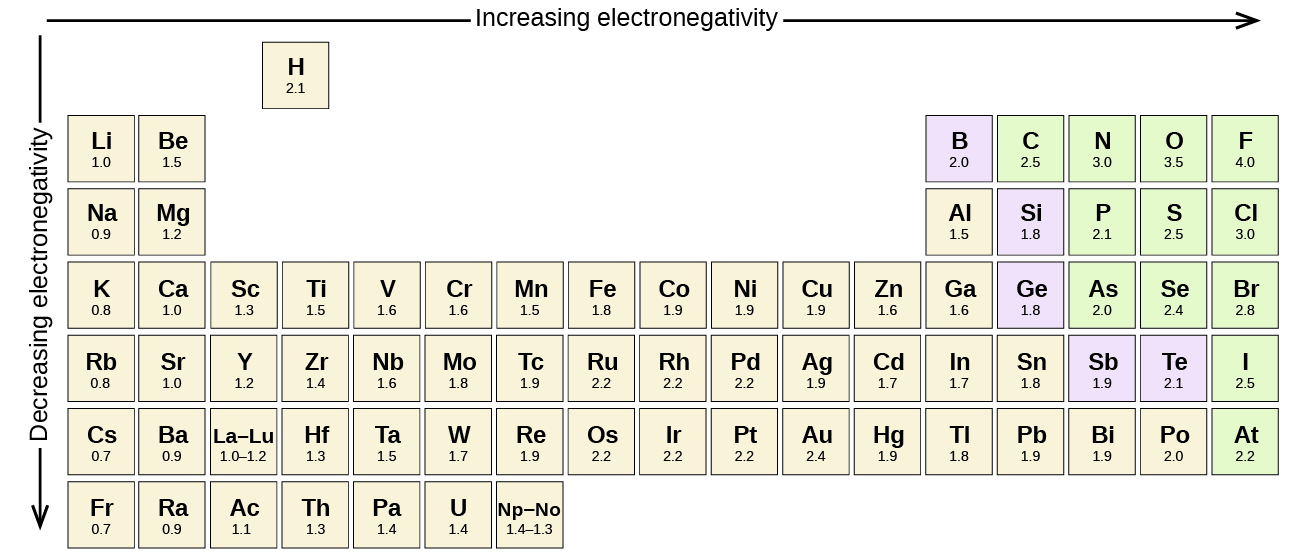
Most of the time the electronegativity values of elements are given in the periodic table. The polarity of a bondthe extent to which it is polaris determined largely by the relative electronegativities of the bonded atoms. A key piece of information they contain is the electronegativity value of each of the elements.
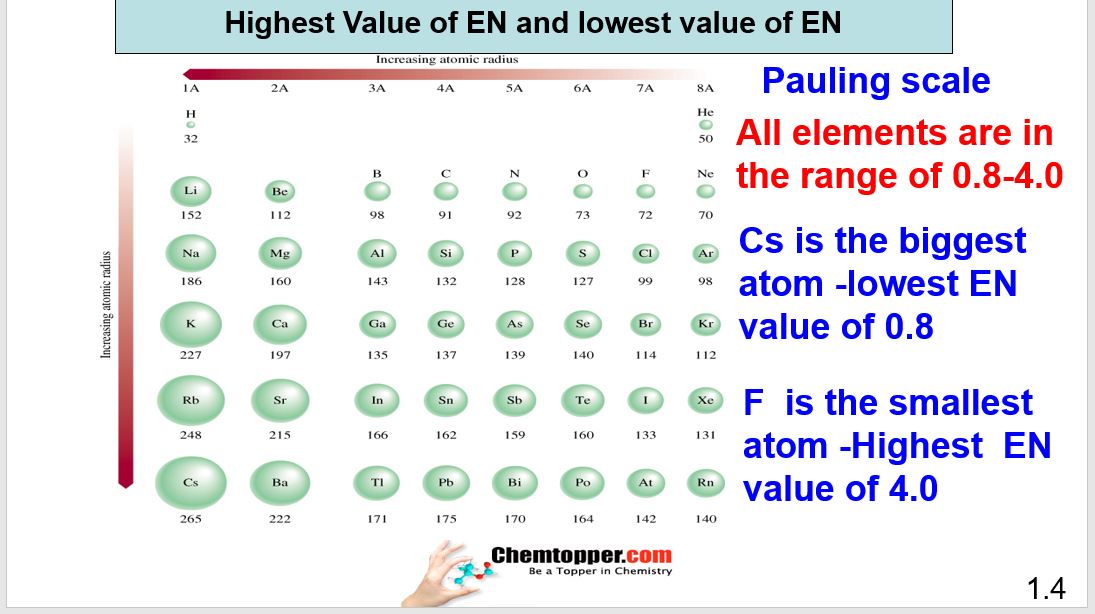
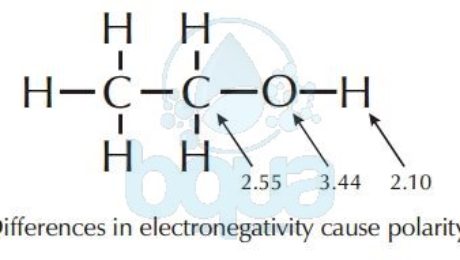
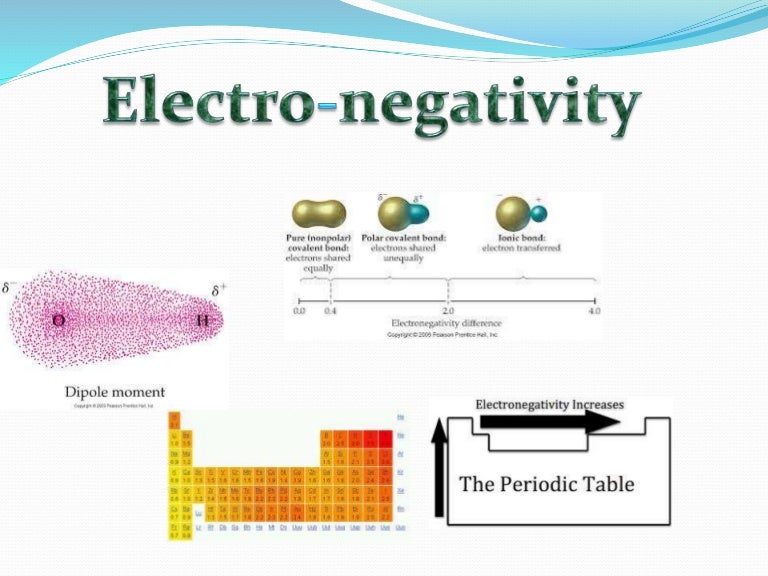
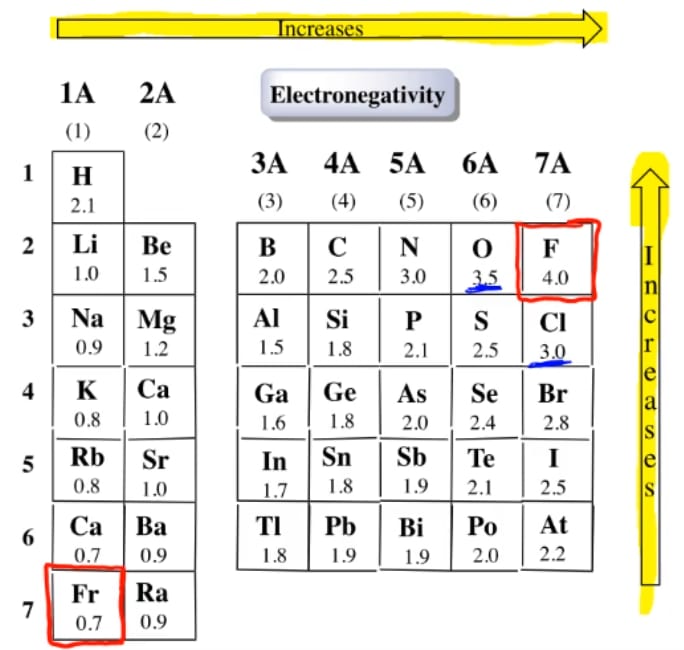
Electronegativity x was defined as the ability of an atom in a molecule or an ion to attract electrons to itself. Thus there is a direct correlation between electronegativity and bond polarity. The values underneath the elements give their electronegativity as measured by the pauling scale.
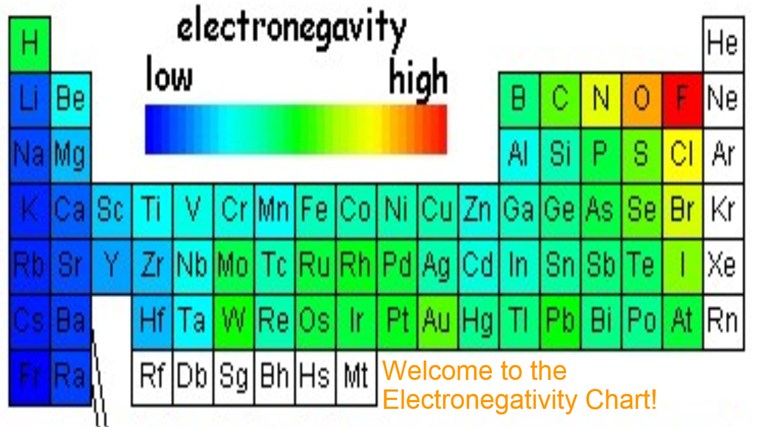
The periodic table contains a lot more information than merely the names of each of the chemical elements. Electronegativity is defined as the ability of an atom in a molecule to attract electrons to itself. Here you can learn about electronegativity of the elements and can also download electronegativity chart in pdf for free.
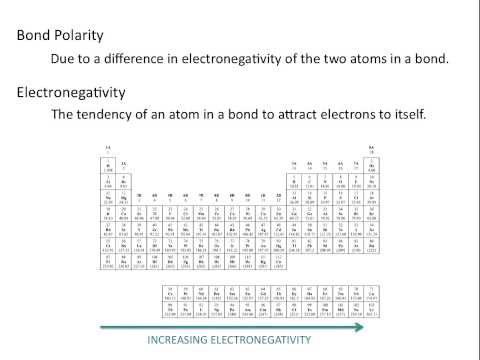
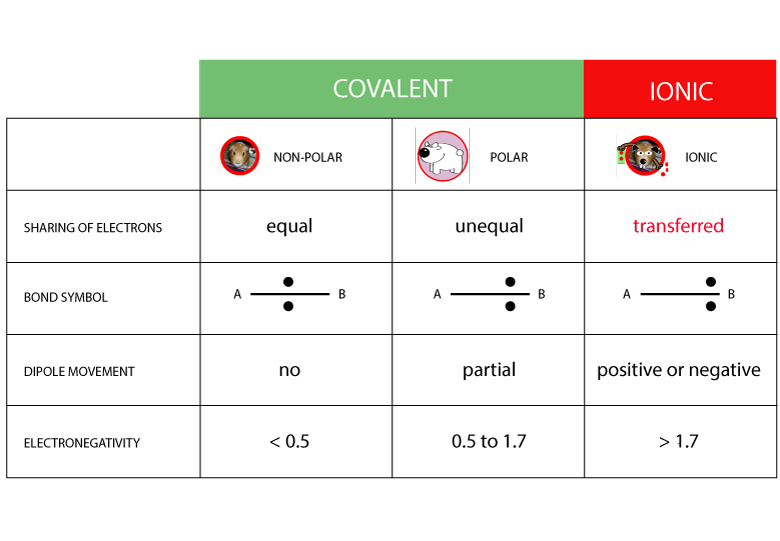
Electronegativity chart and the periodic table. Electronegativity is the strength an atom has to attract a bonding pair of electrons to itself. The electron density that comprises the covalent bond is located halfway between the two atoms.
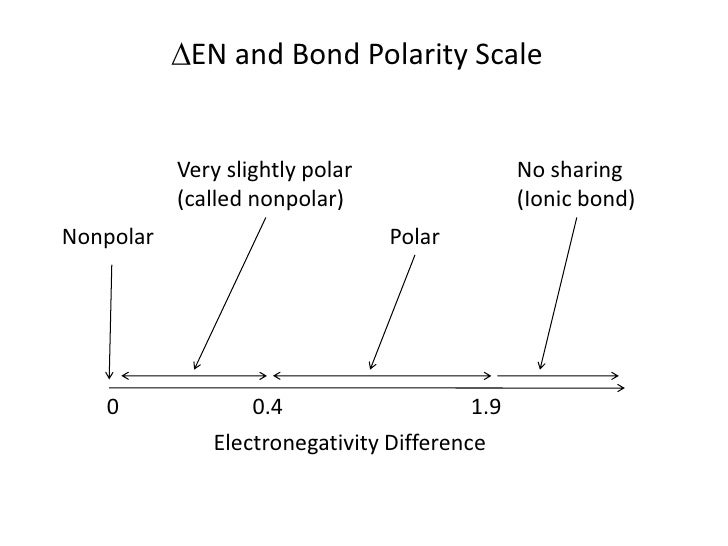
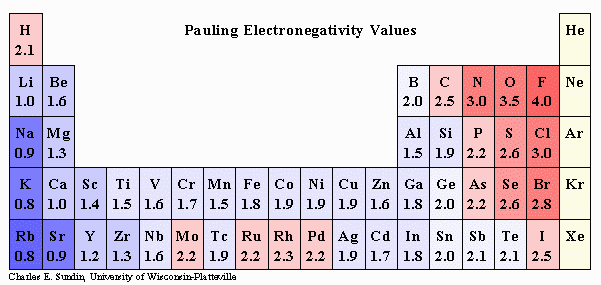
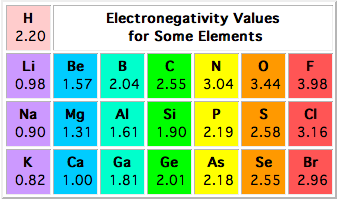
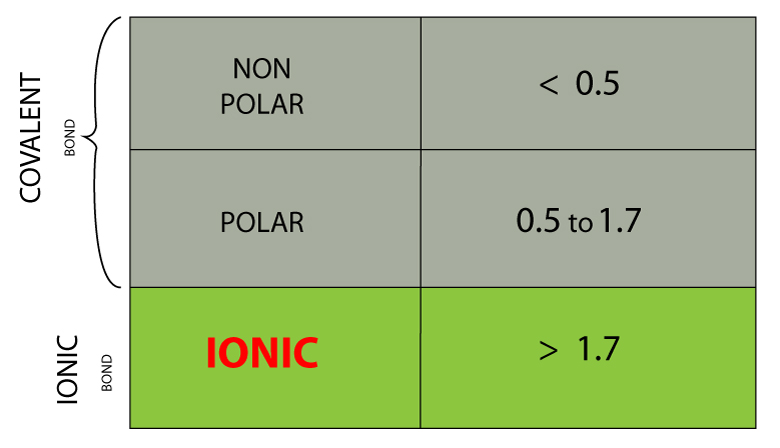
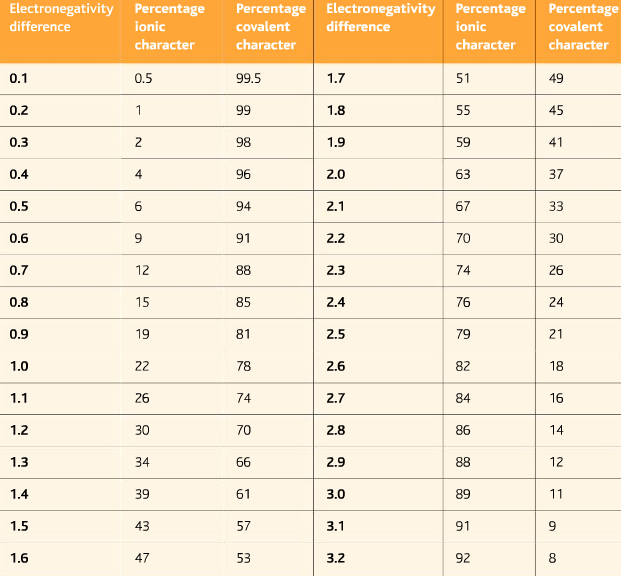
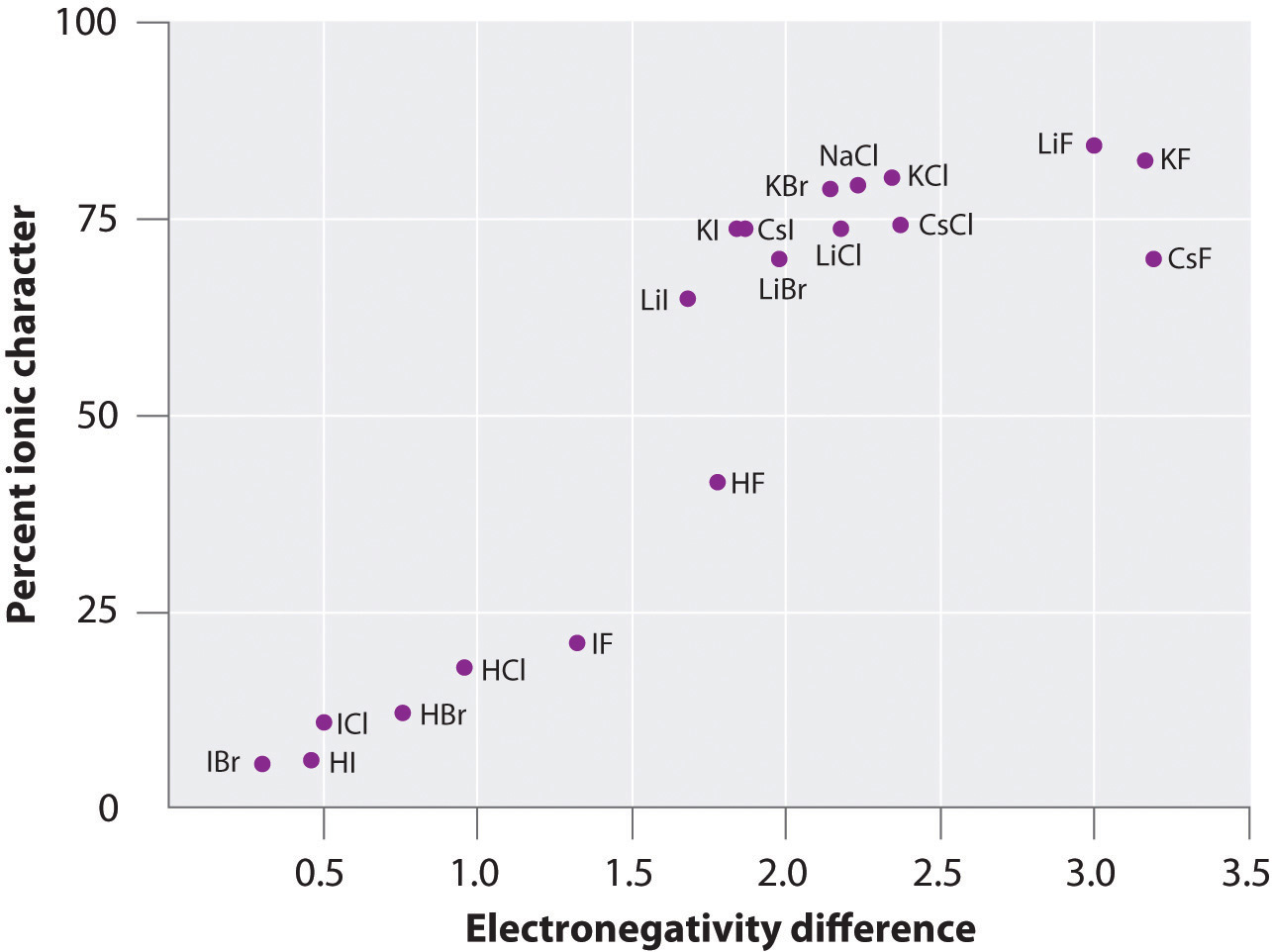
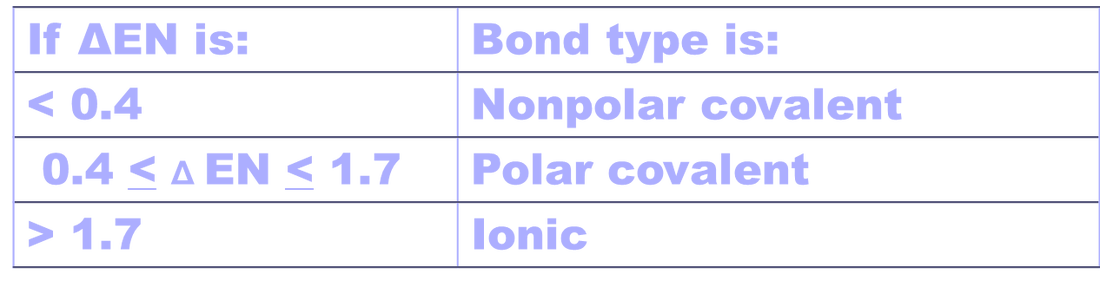
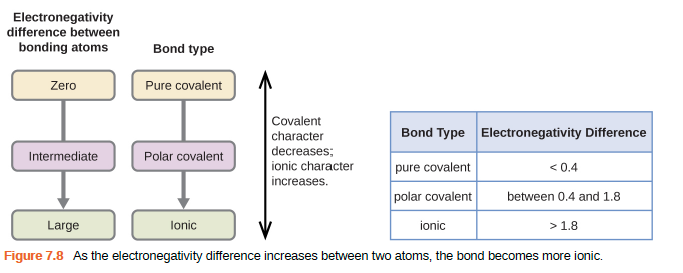
The electronegativity is measured in pauling scale which is from one to four. The absolute value of the difference in electronegativity den of two bonded atoms provides a rough measure of the polarity to be expected in the bond and thus the bond type. Here are the electronegativity values for some common elements.
We use a quantity called electronegativity to estimate whether a given bond is nonpolar covalent polar covalent or ionic. Electronegativity chart here is an electronegativity chart for the elements on the periodic table. Electronegativity is the measure of the ability of an atom to pull the bond pair towards itself when two atoms are involved in a covalent bond.
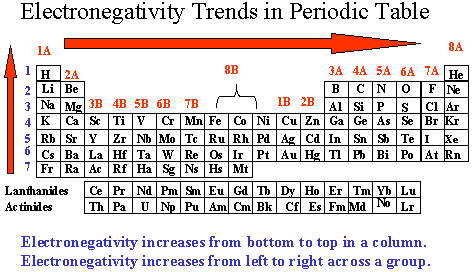
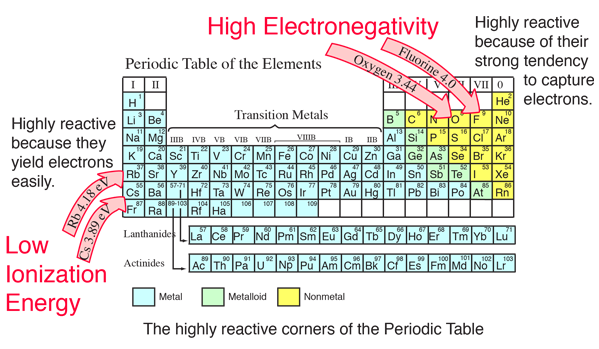
When it is large the bond is polar covalent or ionic. The typical rule is that bonds with an electronegativity difference less than 16 are considered polar. The electronegativity increases across a period and decreases down the group.
The electronegativity chart describes how atoms can attract a pair of electrons to itself by looking at the periodic table you can identify and determine electronegativity values of elements from 0 to 4.

Dublin Schools Lesson Why Are Some Covalent Bonds Polar And Others Nonpolar
dashboard.dublinschools.net
















/PeriodicTableElectronegativity-56a12a045f9b58b7d0bca77c.jpg)